X-rays are electromagnetic waves with a wavelength of approximately 80 to 10 -5 nm. The longest-wavelength X-ray radiation is covered by short-wavelength ultraviolet, the short-wavelength - by long-wavelength γ-radiation. According to the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic.
31.1. DEVICE OF X-RAY TUBE. Bremsstrahlung X-RAY
The most common source of x-rays is the x-ray tube, which is a two-electrode vacuum device (Fig. 31.1). Heated cathode 1 emits electrons 4. Anode 2, often referred to as the anticathode, has an inclined surface in order to direct the resulting X-rays 3 at an angle to the axis of the tube. The anode is made of a highly heat-conducting material to remove the heat generated by the impact of electrons. The anode surface is made of refractory materials having a large atomic number in the periodic table, such as tungsten. In some cases, the anode is specially cooled with water or oil.
For diagnostic tubes, the pinpointness of the X-ray source is important, which can be achieved by focusing electrons in one place of the anticathode. Therefore, constructively, two opposite tasks have to be taken into account: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different parts of the anode. As one of the interesting technical solutions is an X-ray tube with a rotating anode (Fig. 31.2).
As a result of deceleration of an electron (or other charged particle) by the electrostatic field of the atomic nucleus and atomic electrons of the substance of the anticathode, a bremsstrahlung radiation.
Its mechanism can be explained as follows. A moving electric charge is associated with a magnetic field, the induction of which depends on the speed of the electron. When braking, the magnetic
induction and, in accordance with Maxwell's theory, an electromagnetic wave appears.
When electrons decelerate, only part of the energy goes to create an X-ray photon, the other part is spent on heating the anode. Since the ratio between these parts is random, when a large number of electrons decelerate, a continuous spectrum of X-ray radiation is formed. In this regard, bremsstrahlung is also called continuous. On fig. 31.3 shows the dependence of the X-ray flux on the wavelength λ (spectra) at different voltages in the X-ray tube: U 1< U 2 < U 3 .
In each of the spectra, the shortest wavelength bremsstrahlung λ ηίη arises when the energy acquired by an electron in an accelerating field is completely converted into the energy of a photon:

Note that on the basis of (31.2) one of the most accurate methods for the experimental determination of Planck's constant has been developed.
Short-wavelength X-rays usually have a greater penetrating power than long-wavelength ones and are called hard, and longwave soft.
By increasing the voltage on the X-ray tube, the spectral composition of the radiation is changed, as can be seen from Fig. 31.3 and formulas (31.3), and increase the rigidity.
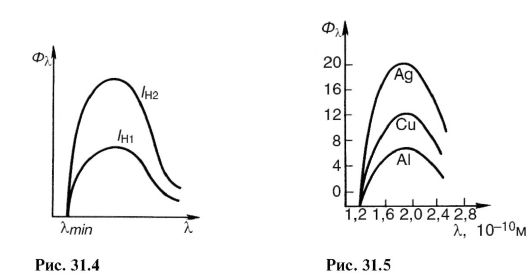
If the cathode filament temperature is increased, then the electron emission and the current in the tube will increase. This will increase the number of X-ray photons emitted every second. Its spectral composition will not change. On fig. 31.4 shows the X-ray bremsstrahlung spectra at the same voltage, but at different cathode filament currents: / n1< / н2 .
The X-ray flux is calculated by the formula:

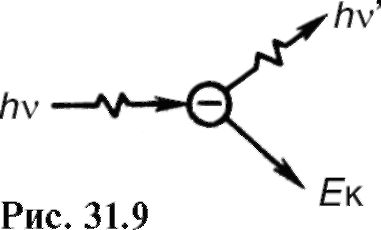
Where U And I- voltage and current in the x-ray tube; Z- serial number of an atom of the anode substance; k- coefficient of proportionality. Spectra obtained from different anticathodes at the same U and I H are shown in fig. 31.5.
31.2. CHARACTERISTIC X-RAY RADIATION. ATOMIC X-RAY SPECTRA
By increasing the voltage on the X-ray tube, one can notice the appearance of a line, which corresponds to
characteristic x-rays(Fig. 31.6). It arises due to the fact that accelerated electrons penetrate deep into the atom and knock out electrons from the inner layers. Electrons from upper levels move to free places (Fig. 31.7), as a result, photons of characteristic radiation are emitted. As can be seen from the figure, the characteristic X-ray radiation consists of series K, L, M etc., the name of which served to designate the electronic layers. Since the emission of the K-series frees up space in the higher layers, the lines of other series are simultaneously emitted.
In contrast to optical spectra, the characteristic x-ray spectra of different atoms are of the same type. On fig. 31.8 shows the spectra of various elements. The uniformity of these spectra is due to the fact that the inner layers of different atoms are the same and differ only energetically, since the force effect from the nucleus increases as the element's atomic number increases. This circumstance leads to the fact that the characteristic spectra shift towards higher frequencies with increasing nuclear charge. This pattern is visible from Fig. 31.8 and known as Moseley's law:

Where v- spectral line frequency; Z- atomic number of the emitting element; A And IN- permanent.
There is another difference between optical and x-ray spectra.
The characteristic X-ray spectrum of an atom does not depend on the chemical compound in which this atom is included. For example, the X-ray spectrum of the oxygen atom is the same for O, O 2 and H 2 O, while the optical spectra of these compounds are significantly different. This feature of the x-ray spectrum of the atom was the basis for the name characteristic.
Characteristic radiation always occurs when there is free space in the inner layers of an atom, regardless of the reason that caused it. So, for example, characteristic radiation accompanies one of the types of radioactive decay (see 32.1), which consists in the capture of an electron from the inner layer by the nucleus.

31.3. INTERACTION OF X-RAY RADIATION WITH SUBSTANCE
The registration and use of X-ray radiation, as well as its impact on biological objects, are determined by the primary processes of interaction of an X-ray photon with electrons of atoms and molecules of a substance.
Depending on the ratio of energy hv photon and ionization energy 1 A and there are three main processes.
Coherent (classical) scattering
Scattering of long-wavelength X-rays occurs mainly without a change in wavelength, and is called coherent. It occurs if the photon energy is less than the ionization energy: hv< A and.
Since in this case the energy of the X-ray photon and the atom does not change, coherent scattering in itself does not cause a biological effect. However, when creating protection against X-ray radiation, one should take into account the possibility of changing the direction of the primary beam. This kind of interaction is important for X-ray diffraction analysis (see 24.7).
Incoherent scattering (Compton effect)
In 1922 A.Kh. Compton, observing the scattering of hard X-rays, discovered a decrease in the penetrating power of the scattered beam compared to the incident beam. This meant that the wavelength of the scattered X-rays was greater than that of the incident X-rays. The scattering of X-rays with a change in wavelength is called incoherent nym, and the phenomenon itself - the Compton effect. It occurs if the energy of the X-ray photon is greater than the ionization energy: hv > A and.
This phenomenon is due to the fact that when interacting with an atom, the energy hv photon is spent on the production of a new scattered X-ray photon with energy hv", to detach an electron from an atom (ionization energy A u) and impart kinetic energy to the electron E to:
hv \u003d hv " + A and + E k.(31.6)
1 Here, ionization energy is understood as the energy required to remove internal electrons from an atom or molecule.
Since in many cases hv>> A and and the Compton effect occurs on free electrons, then we can write approximately:
hv = hv"+ E K .(31.7)
It is significant that in this phenomenon (Fig. 31.9), along with secondary X-ray radiation (energy hv" photon) recoil electrons appear (kinetic energy E to electron). Atoms or molecules then become ions.
photoelectric effect
In the photoelectric effect, X-ray radiation is absorbed by an atom, as a result of which an electron flies out, and the atom is ionized (photoionization).
The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. For example, ionized atoms can emit a characteristic spectrum, excited atoms can become sources of visible light (X-ray luminescence), etc.
On fig. 31.10 is a diagram of the possible processes that occur when X-ray radiation enters a substance. Several tens of processes similar to the one shown may occur before the energy of the X-ray photon is converted into the energy of molecular thermal motion. As a result, there will be changes in the molecular composition of the substance.
The processes represented by the diagram in fig. 31.10, underlie the phenomena observed under the action of X-rays on matter. Let's list some of them.
X-ray luminescence- the glow of a number of substances under x-ray irradiation. Such a glow of platinum-cyanogen barium allowed Roentgen to discover the rays. This phenomenon is used to create special luminous screens for the purpose of visual observation of x-rays, sometimes to enhance the action of x-rays on a photographic plate.
The chemical action of X-ray radiation is known, for example, the formation of hydrogen peroxide in water. A practically important example is the effect on a photographic plate, which makes it possible to detect such rays.
The ionizing effect is manifested in an increase in electrical conductivity under the influence of X-rays. This property is used

in dosimetry to quantify the effect of this type of radiation.
As a result of many processes, the primary X-ray beam is weakened in accordance with the law (29.3). Let's write it in the form:
I = I0 e-/", (31.8)
Where μ - linear attenuation coefficient. It can be represented as consisting of three terms corresponding to coherent scattering μ κ , incoherent μ ΗΚ and photoeffect μ f:
μ = μ k + μ hk + μ f. (31.9)
The intensity of X-ray radiation is attenuated in proportion to the number of atoms of the substance through which this flow passes. If we compress matter along the axis x, for example, in b times by increasing b times its density, then

31.4. PHYSICAL FOUNDATIONS OF THE APPLICATION OF X-RAY IN MEDICINE
One of the most important medical applications of X-rays is the transillumination of internal organs for diagnostic purposes. (X-ray diagnostics).
For diagnostics, photons with an energy of about 60-120 keV are used. At this energy, the mass extinction coefficient is mainly determined by the photoelectric effect. Its value is inversely proportional to the third power of the photon energy (proportional to λ 3), which manifests a large penetrating power of hard radiation, and proportional to the third power of the atomic number of the absorbing substance:

A significant difference in the absorption of x-ray radiation by different tissues allows you to see images of the internal organs of the human body in a shadow projection.
X-ray diagnostics is used in two versions: fluoroscopy the image is viewed on an X-ray luminescent screen, radiography - the image is fixed on the film.
If the organ under study and the surrounding tissues attenuate x-rays approximately equally, then special contrast agents are used. So, for example, filling the stomach and intestines with a mushy mass of barium sulfate, one can see their shadow image.
The brightness of the image on the screen and the exposure time on the film depend on the intensity of the x-rays. If it is used for diagnosis, then the intensity cannot be high, so as not to cause undesirable biological consequences. Therefore, there are a number of technical devices that improve the image at low X-ray intensities. An example of such a device is intensifier tubes (see 27.8). In a mass examination of the population, a variant of radiography is widely used - fluorography, in which an image from a large X-ray luminescent screen is recorded on a sensitive small-format film. When shooting, a lens of large aperture is used, the finished pictures are examined on a special magnifier.

An interesting and promising option for radiography is a method called x-ray tomography, and its "machine version" - CT scan.
Let's consider this question.
A plain radiograph covers a large area of the body, with various organs and tissues shading each other. You can avoid this if you periodically move the X-ray tube together (Fig. 31.11) in antiphase RT and film Fp relative to the object About research. The body contains a number of inclusions that are opaque to X-rays; they are shown by circles in the figure. As you can see, x-rays at any position of the x-ray tube (1, 2 etc.) pass through
cutting the same point of the object, which is the center, relative to which the periodic movement is performed RT And Fp. This point, more precisely a small opaque inclusion, is shown by a dark circle. His shadow image moves with fp, occupying successively positions 1, 2 etc. The remaining inclusions in the body (bones, seals, etc.) create on Fp some general background, since x-rays are not permanently obscured by them. By changing the position of the swing center, it is possible to obtain a layer-by-layer X-ray image of the body. Hence the name - tomography(layered recording).
It is possible, using a thin X-ray beam, to screen (instead of Fp), consisting of semiconductor detectors of ionizing radiation (see 32.5), and a computer, to process the shadow x-ray image in tomography. This modern version of tomography (computed or computed x-ray tomography) allows you to get layered images of the body on the screen of a cathode ray tube or on paper with details of less than 2 mm with a difference in x-ray absorption of up to 0.1%. This allows, for example, to distinguish between the gray and white matter of the brain and to see very small tumor formations.
1. X-ray sources.
2. Bremsstrahlung X-rays.
3. Characteristic x-ray radiation. Moseley's law.
4. Interaction of X-ray radiation with matter. The law of weakening.
5. Physical basis for the use of X-rays in medicine.
6. Basic concepts and formulas.
7. Tasks.
X-ray radiation - electromagnetic waves with a wavelength from 100 to 10 -3 nm. On the scale of electromagnetic waves, X-ray radiation occupies the region between UV radiation and γ -radiation. X-rays (X-rays) were discovered in 1895 by K. Roentgen, who in 1901 became the first Nobel laureate in physics.
32.1. X-ray sources
Natural sources of X-rays are some radioactive isotopes (for example, 55 Fe). Artificial sources of powerful X-rays are x-ray tubes(Fig. 32.1).
Rice. 32.1. X-ray tube device
The X-ray tube is an evacuated glass flask with two electrodes: the anode A and the cathode K, between which a high voltage U (1-500 kV) is created. The cathode is a coil heated by electric current. Electrons emitted by a heated cathode (thermionic emission) are accelerated by an electric field to big speeds (for this you need high voltage) and fall on the anode of the tube. When these electrons interact with the anode material, two types of X-ray radiation arise: brake And characteristic.
The working surface of the anode is located at some angle to the direction of the electron beam in order to create the desired direction of the x-rays.
Approximately 1% of the kinetic energy of electrons is converted into X-rays. The rest of the energy is released as heat. Therefore, the working surface of the anode is made of a refractory material.
32.2. Bremsstrahlung X-ray
An electron moving in some medium loses its speed. This creates a negative acceleration. According to Maxwell's theory, any accelerated the movement of a charged particle is accompanied by electromagnetic radiation. The radiation that occurs when an electron decelerates in the anode material is called bremsstrahlung X-rays.
The properties of bremsstrahlung are determined by the following factors.
1. Radiation is emitted by individual quanta, the energies of which are related to the frequency by the formula (26.10)
![]() where ν is the frequency, λ is the wavelength.
where ν is the frequency, λ is the wavelength.
2. All electrons reaching the anode have the same kinetic energy equal to the work of the electric field between the anode and cathode:
where e is the electron charge, U is the accelerating voltage.
3. The kinetic energy of an electron is partially transferred to the substance and goes to heat it (Q), and is partially spent on the creation of an X-ray quantum:
4. Relationship between Q and hv accidentally.
Due to the last property (4), the quanta generated by various electrons, have various frequencies and wavelengths. Therefore, the bremsstrahlung spectrum is solid. typical view spectral density the X-ray flux (Φ λ = άΦ/άλ) is shown in fig. 32.2.
 Rice. 32.2. Bremsstrahlung spectrum
Rice. 32.2. Bremsstrahlung spectrum
From the side of long waves, the spectrum is limited by a wavelength of 100 nm, which is the boundary of X-ray radiation. From the side of short waves, the spectrum is limited by the wavelength λ min . According to formula (32.2) minimum wavelength corresponds to the case Q = 0 (the kinetic energy of the electron is completely converted into the energy of the quantum):
 Calculations show that the bremsstrahlung flux (Φ) is directly proportional to the square of the voltage U between
Calculations show that the bremsstrahlung flux (Φ) is directly proportional to the square of the voltage U between
anode and cathode, current I in the tube and atomic number Z of the anode substance:
The X-ray bremsstrahlung spectra at various voltages, various cathode temperatures, and various anode materials are shown in Figs. 32.3.
 Rice. 32.3. Bremsstrahlung spectrum (Φ λ):
Rice. 32.3. Bremsstrahlung spectrum (Φ λ):
a - at different voltages U in the tube; b - at different temperatures T
cathode; c - with different anode substances differing in parameter Z
With an increase in the anode voltage, the value λmin shifts towards shorter wavelengths. At the same time, the height of the spectral curve also increases (Fig. 32.3, A).
As the cathode temperature increases, the electron emission increases. Correspondingly, the current I in the tube also increases. The height of the spectral curve increases, but the spectral composition of the radiation does not change (Fig. 32.3, b).
When the anode material changes, the height of the spectral curve changes in proportion to the atomic number Z (Fig. 32.3, c).
32.3. Characteristic x-ray radiation. Moseley's law
When cathode electrons interact with anode atoms, along with X-ray bremsstrahlung, X-ray radiation arises, the spectrum of which consists of individual lines. This radiation
has the following origin. Some cathodic electrons penetrate deep into the atom and knock electrons out of it. inner shells. The vacancies thus formed are filled with electrons with top shells, resulting in the emission of radiation quanta. This radiation contains a discrete set of frequencies determined by the anode material and is called characteristic radiation. The full spectrum of an x-ray tube is a superposition of the characteristic spectrum on the bremsstrahlung spectrum (Fig. 32.4).
 Rice. 32.4. X-ray tube emission spectrum
Rice. 32.4. X-ray tube emission spectrum
The existence of characteristic X-ray spectra has been discovered using X-ray tubes. Later it was found that such spectra arise during any ionization of the inner orbits of chemical elements. Having studied the characteristic spectra of various chemical elements, G. Moseley (1913) established the following law, which bears his name.
The square root of the frequency of the characteristic radiation is a linear function of the ordinal number of the element:
where ν is the frequency of the spectral line, Z is the atomic number of the emitting element, A, B are constants.
Moseley's law makes it possible to determine the atomic number of a chemical element from the observed spectrum of characteristic radiation. This played a big role in the placement of elements in the periodic system.
32.4. Interaction of X-ray radiation with matter. law of weakening
There are two main types of interaction of X-ray radiation with matter: scattering and photoelectric effect. When scattered, the direction of motion of a photon changes. In the photoelectric effect, a photon absorbed.
1. Coherent (elastic) scattering occurs when the energy of an X-ray photon is insufficient for the internal ionization of an atom (knocking out an electron from one of the inner shells). In this case, the direction of motion of the photon changes, and its energy and wavelength do not change (therefore, this scattering is called elastic).
 2. Incoherent (Compton) scattering occurs when the photon energy is much greater than the internal ionization energy A u: hv >> A u.
2. Incoherent (Compton) scattering occurs when the photon energy is much greater than the internal ionization energy A u: hv >> A u.
In this case, the electron breaks away from the atom and acquires some kinetic energy E k. The direction of the photon during Compton scattering changes, and its energy decreases:
Compton scattering is associated with the ionization of the atoms of matter.
 3. photoelectric effect occurs when the photon energy hv is sufficient to ionize the atom: hv > A u. At the same time, the X-ray quantum absorbed and its energy is spent on the ionization of the atom and the communication of kinetic energy to the ejected electron E k \u003d hv - AI.
3. photoelectric effect occurs when the photon energy hv is sufficient to ionize the atom: hv > A u. At the same time, the X-ray quantum absorbed and its energy is spent on the ionization of the atom and the communication of kinetic energy to the ejected electron E k \u003d hv - AI.
 Compton scattering and the photoelectric effect are accompanied by characteristic X-ray radiation, since after the knocking out of internal electrons, the vacancies are filled with electrons from the outer shells.
Compton scattering and the photoelectric effect are accompanied by characteristic X-ray radiation, since after the knocking out of internal electrons, the vacancies are filled with electrons from the outer shells.
X-ray luminescence. In some substances, electrons and quanta of Compton scattering, as well as photoelectric effect electrons, cause excitation of molecules, which is accompanied by radiative transitions to the ground state. This produces a glow called X-ray luminescence. The luminescence of barium platinum-cyanogen allowed X-rays to be discovered by Roentgen.
law of weakening
The scattering of X-rays and the photoelectric effect lead to the fact that as the X-ray radiation penetrates deep into the primary beam of radiation is weakened (Fig. 32.5). The easing is exponential:
 The value of μ depends on the absorbing material and the radiation spectrum. For practical calculations, as a characteristic of the weakened
The value of μ depends on the absorbing material and the radiation spectrum. For practical calculations, as a characteristic of the weakened
 Rice. 32.5. Attenuation of the X-ray flux in the direction of the incident rays
Rice. 32.5. Attenuation of the X-ray flux in the direction of the incident rays
 Where λ
- wavelength; Z is the atomic number of the element; k is some constant.
Where λ
- wavelength; Z is the atomic number of the element; k is some constant.
32.5. Physical bases of use
x-ray radiation in medicine
In medicine, X-rays are used for diagnostic and therapeutic purposes.
X-ray diagnostics- Methods for obtaining images of internal organs using x-rays.
The physical basis of these methods is the law of X-ray attenuation in matter (32.10). Cross-sectional uniform X-ray flux after passing through inhomogeneous tissue will become inhomogeneous. This inhomogeneity can be recorded on photographic film, a fluorescent screen, or using a matrix photodetector. For example, the mass weakening coefficients of bone tissue - Ca 3 (PO 4) 2 - and soft tissues - mainly H 2 O - differ by 68 times (μ m bone /μ m water = 68). Bone density is also higher than soft tissue density. Therefore, an x-ray image produces a light image of the bone against a darker background of soft tissues.
If the organ under study and the tissues surrounding it have similar attenuation coefficients, then special contrast agents. So, for example, during fluoroscopy of the stomach, the subject takes a mushy mass of barium sulfate (BaSO 4), in which the mass attenuation coefficient is 354 times greater than that of soft tissues.
For diagnostics, X-ray radiation with a photon energy of 60-120 keV is used. In medical practice, the following methods of X-ray diagnostics are used.
1. X-ray. The image is formed on a fluorescent screen. The image brightness is low and can only be viewed in a darkened room. The physician must be protected from exposure.
The advantage of fluoroscopy is that it is carried out in real time. The disadvantage is a large radiation load on the patient and the doctor (compared to other methods).
The modern version of fluoroscopy - X-ray television - uses X-ray image intensifiers. The amplifier perceives the weak glow of the X-ray screen, amplifies it and transmits it to the TV screen. As a result, the radiation load on the doctor has sharply decreased, the brightness of the image has increased, and it has become possible to record the results of the examination on video.
2. Radiography. The image is formed on a special film that is sensitive to x-rays. Pictures are taken in two mutually perpendicular projections (direct and lateral). The image becomes visible after photo processing. The finished dried image is viewed in transmitted light.
At the same time, details are satisfactorily visible, the contrast of which differs by 1-2%.
In some cases, before the examination, the patient is given a special contrast agent. For example, an iodine-containing solution (intravenously) in the study of the kidneys and urinary tract.
The advantages of radiography are high resolution, short exposure time and almost complete safety for the doctor. The disadvantages include the static image (the object cannot be traced in dynamics).
3. Fluorography. In this examination, the image obtained on the screen is photographed on a sensitive small format film. Fluorography is widely used in the mass survey of the population. If pathological changes are found on the fluorogram, then the patient is prescribed a more detailed examination.
4. Electroroentgenography. This type of examination differs from conventional radiography in the way the image is captured. Use instead of film selenium plate, electrified by X-rays. The result is a latent image of electrical charges that can be made visible and transferred to paper.
5. Angiography. This method is used in the examination of blood vessels. A contrast agent is injected into the vein through a catheter, after which a powerful x-ray machine takes a series of images following each other in a fraction of a second. Figure 32.6 shows an angiogram in the region of the carotid artery.
6. X-ray computed tomography. This type of X-ray examination allows you to get an image of a flat section of the body with a thickness of several mm. In this case, the given section is repeatedly illuminated at different angles with the fixation of each individual image in the computer's memory. Then
 Rice. 32.6. Angiogram showing a narrowing in the canal of the carotid artery
Rice. 32.6. Angiogram showing a narrowing in the canal of the carotid artery
 Rice. 32.7. Scanning scheme of tomography (a); tomogram of the head in cross section at eye level (b).
Rice. 32.7. Scanning scheme of tomography (a); tomogram of the head in cross section at eye level (b).
computer reconstruction is carried out, the result of which is the image of the scanned layer (Fig. 32.7).
Computed tomography makes it possible to distinguish elements with a density difference between them up to 1%. Conventional radiography allows you to capture a minimum difference in density between adjacent areas of 10-20%.
X-ray therapy - the use of x-rays to destroy malignant tumors.
The biological effect of radiation is to disrupt the vital activity of especially rapidly multiplying cells. Very hard X-rays (with a photon energy of approximately 10 MeV) are used to destroy cancer cells deep within the body. To reduce damage to healthy surrounding tissues, the beam rotates around the patient in such a way that only the damaged area remains under its influence at all times.
32.6. Basic concepts and formulas
 Table continuation
Table continuation
 End of table
End of table
 32.7. Tasks
32.7. Tasks
1. Why does an electron beam in medical X-ray tubes strike one point of the anticathode, and does not fall on it in a wide beam?
Answer: to obtain a point source of x-rays, giving a sharp outline of translucent objects on the screen.
2. Find the boundary of bremsstrahlung X-rays (frequency and wavelength) for voltages U 1 = 2 kV and U 2 = 20 kV.
 4.
Lead screens are used to protect against x-rays. The linear absorption of X-rays in lead is 52 cm -1 . What should be the thickness of the shielding layer of lead in order for it to reduce the X-ray intensity by 30 times?
4.
Lead screens are used to protect against x-rays. The linear absorption of X-rays in lead is 52 cm -1 . What should be the thickness of the shielding layer of lead in order for it to reduce the X-ray intensity by 30 times?
 5.
Find the X-ray tube radiation flux at U = 50 kV, I = 1 mA. The anode is made of tungsten (Z = 74). Find the efficiency of the tube.
5.
Find the X-ray tube radiation flux at U = 50 kV, I = 1 mA. The anode is made of tungsten (Z = 74). Find the efficiency of the tube.
 6.
For X-ray diagnostics of soft tissues, contrast agents are used. For example, the stomach and intestines are filled with a mass of barium sulfate (BaSO 4 ). Compare the mass attenuation coefficients of barium sulfate and soft tissues (water).
6.
For X-ray diagnostics of soft tissues, contrast agents are used. For example, the stomach and intestines are filled with a mass of barium sulfate (BaSO 4 ). Compare the mass attenuation coefficients of barium sulfate and soft tissues (water).
 7. What will give a thicker shadow on the X-ray screen: aluminum (Z = 13, ρ = 2.7 g/cm 3) or the same layer of copper (Z = 29, ρ = 8.9 g/cm 3)?
7. What will give a thicker shadow on the X-ray screen: aluminum (Z = 13, ρ = 2.7 g/cm 3) or the same layer of copper (Z = 29, ρ = 8.9 g/cm 3)?
 8.
How many times is the thickness of the aluminum layer greater than the thickness of the copper layer, if the layers attenuate x-rays in the same way?
8.
How many times is the thickness of the aluminum layer greater than the thickness of the copper layer, if the layers attenuate x-rays in the same way?
X-rays play one of the most important roles in the study and practical use of atomic phenomena. Thanks to their research, many discoveries were made and methods for analyzing substances were developed, which are used in various fields. Here we will consider one of the types of X-rays - characteristic X-rays.
Nature and properties of X-rays
X-ray radiation is a high-frequency change in the state of an electromagnetic field propagating in space at a speed of about 300,000 km / s, that is, electromagnetic waves. On the scale of the range of electromagnetic radiation, X-rays are located in the wavelength range from approximately 10 -8 to 5∙10 -12 meters, which is several orders of magnitude shorter than optical waves. This corresponds to frequencies from 3∙10 16 to 6∙10 19 Hz and energies from 10 eV to 250 keV, or 1.6∙10 -18 to 4∙10 -14 J. It should be noted that the boundaries of the frequency ranges of electromagnetic radiation are rather arbitrary due to their overlap.
Is the interaction of accelerated charged particles (high-energy electrons) with electric and magnetic fields and with atoms of matter.
X-ray photons are characterized by high energies and high penetrating and ionizing power, especially for hard X-rays with wavelengths less than 1 nanometer (10 -9 m).
X-rays interact with matter, ionizing its atoms, in the processes of the photoelectric effect (photoabsorption) and incoherent (Compton) scattering. In photoabsorption, an X-ray photon, being absorbed by an electron of an atom, transfers energy to it. If its value exceeds the binding energy of an electron in an atom, then it leaves the atom. Compton scattering is characteristic of harder (energetic) X-ray photons. Part of the energy of the absorbed photon is spent on ionization; in this case, at a certain angle to the direction of the primary photon, a secondary one is emitted, with a lower frequency.
Types of X-ray radiation. Bremsstrahlung
To obtain rays, glass vacuum bottles with electrodes located inside are used. The potential difference across the electrodes needs to be very high - up to hundreds of kilovolts. On a tungsten cathode heated by current, thermionic emission occurs, that is, electrons are emitted from it, which, accelerated by the potential difference, bombard the anode. As a result of their interaction with the atoms of the anode (sometimes called the anticathode), X-ray photons are born.
Depending on what process leads to the birth of a photon, there are such types of X-ray radiation as bremsstrahlung and characteristic.
Electrons can, meeting with the anode, slow down, that is, lose energy in the electric fields of its atoms. This energy is emitted in the form of X-ray photons. Such radiation is called bremsstrahlung.
It is clear that the braking conditions will differ for individual electrons. This means that different amounts of their kinetic energy are converted into X-rays. As a result, bremsstrahlung includes photons of different frequencies and, accordingly, wavelengths. Therefore, its spectrum is continuous (continuous). Sometimes for this reason it is also called "white" X-rays.
The energy of the bremsstrahlung photon cannot exceed the kinetic energy of the electron that generates it, so that the maximum frequency (and the smallest wavelength) of bremsstrahlung corresponds to the largest value of the kinetic energy of electrons incident on the anode. The latter depends on the potential difference applied to the electrodes.
There is another type of X-ray that comes from a different process. This radiation is called characteristic, and we will dwell on it in more detail.
How characteristic X-rays are produced
Having reached the anticathode, a fast electron can penetrate inside the atom and knock out any electron from one of the lower orbitals, that is, transfer to it energy sufficient to overcome the potential barrier. However, if there are higher energy levels occupied by electrons in the atom, the vacated place will not remain empty.
It must be remembered that the electronic structure of the atom, like any energy system, seeks to minimize energy. The vacancy formed as a result of the knockout is filled with an electron from one of the higher levels. Its energy is higher, and, occupying a lower level, it radiates a surplus in the form of a quantum of characteristic X-ray radiation.
The electronic structure of an atom is a discrete set of possible energy states of electrons. Therefore, X-ray photons emitted during the replacement of electron vacancies can also have only strictly defined energy values, reflecting the level difference. As a result, the characteristic X-ray radiation has a spectrum not of a continuous, but of a line type. Such a spectrum makes it possible to characterize the substance of the anode - hence the name of these rays. It is precisely because of the spectral differences that it is clear what is meant by bremsstrahlung and characteristic X-rays.
Sometimes the excess energy is not emitted by the atom, but is spent on knocking out the third electron. This process - the so-called Auger effect - is more likely to occur when the electron binding energy does not exceed 1 keV. The energy of the released Auger electron depends on the structure of the energy levels of the atom, so the spectra of such electrons are also discrete.
General view of the characteristic spectrum
Narrow characteristic lines are present in the X-ray spectral pattern along with a continuous bremsstrahlung spectrum. If we represent the spectrum as a plot of intensity versus wavelength (frequency), we will see sharp peaks at the locations of the lines. Their position depends on the anode material. These maxima are present at any potential difference - if there are X-rays, there are always peaks too. With increasing voltage at the electrodes of the tube, the intensity of both continuous and characteristic X-ray radiation increases, but the location of the peaks and the ratio of their intensities does not change.

The peaks in the X-ray spectra have the same shape regardless of the material of the anti-cathode irradiated by electrons, but for different materials they are located at different frequencies, uniting in series according to the proximity of the frequency values. Between the series themselves, the difference in frequencies is much more significant. The shape of the maxima does not depend in any way on whether the anode material represents a pure chemical element or whether it is a complex substance. In the latter case, the characteristic X-ray spectra of its constituent elements are simply superimposed on each other.
With an increase in the atomic number of a chemical element, all lines of its X-ray spectrum are shifted towards increasing frequency. The spectrum retains its form.
Moseley's law
The phenomenon of spectral shift of characteristic lines was experimentally discovered by the English physicist Henry Moseley in 1913. This allowed him to associate the frequencies of the maxima of the spectrum with the ordinal numbers of the chemical elements. Thus, the wavelength of the characteristic X-ray radiation, as it turned out, can be clearly correlated with a specific element. In general terms, Moseley's law can be written as follows: √f = (Z - S n)/n√R, where f is the frequency, Z is the element's ordinal number, S n is the screening constant, n is the principal quantum number, and R is the Rydberg constant. This relationship is linear and appears on the Moseley diagram as a series of straight lines for each value of n.
The values of n correspond to individual series of characteristic X-ray peaks. Moseley's law allows one to determine the serial number of a chemical element irradiated by hard electrons from the measured wavelengths (they are uniquely related to the frequencies) of the X-ray spectrum maxima.
The structure of the electron shells of chemical elements is identical. This is indicated by the monotonicity of the shift change in the characteristic spectrum of X-rays. The frequency shift reflects not structural, but energy differences between electron shells, unique for each element.

The role of Moseley's law in atomic physics
There are small deviations from the strict linear relationship expressed by Moseley's law. They are connected, firstly, with the peculiarities of the filling order of the electron shells in some elements, and, secondly, with the relativistic effects of the motion of electrons in heavy atoms. In addition, when the number of neutrons in the nucleus changes (the so-called isotopic shift), the position of the lines can change slightly. This effect made it possible to study the atomic structure in detail.
The significance of Moseley's law is extremely great. Its consistent application to the elements of Mendeleev's periodic system established the pattern of increasing the serial number according to each small shift in the characteristic maxima. This contributed to the clarification of the question of the physical meaning of the ordinal number of elements. The Z value is not just a number: it is the positive electric charge of the nucleus, which is the sum of the unit positive charges of the particles that make up it. The correct placement of elements in the table and the presence of empty positions in it (then they still existed) received powerful confirmation. The validity of the periodic law was proved.
Moseley's law, in addition, became the basis on which a whole area of experimental research arose - X-ray spectrometry.
The structure of the electron shells of the atom
Let us briefly recall how the electronic structure is arranged. It consists of shells, denoted by the letters K, L, M, N, O, P, Q, or numbers from 1 to 7. Electrons within the shell are characterized by the same main quantum number n, which determines the possible energy values. In outer shells, the energy of electrons is higher, and the ionization potential for outer electrons is correspondingly lower.
The shell includes one or more sublevels: s, p, d, f, g, h, i. In each shell, the number of sublevels increases by one compared to the previous one. The number of electrons in each sublevel and in each shell cannot exceed a certain value. They are characterized, in addition to the main quantum number, by the same value of the orbital electron cloud that determines the shape. Sublevels are labeled with the shell they belong to, such as 2s, 4d, and so on.
The sublevel contains which are set, in addition to the main and orbital, by one more quantum number - magnetic, which determines the projection of the electron's orbital momentum onto the direction of the magnetic field. One orbital can have no more than two electrons, differing in the value of the fourth quantum number - spin.

Let us consider in more detail how characteristic X-ray radiation arises. Since the origin of this type of electromagnetic emission is associated with phenomena occurring inside the atom, it is most convenient to describe it precisely in the approximation of electronic configurations.
The mechanism of generation of characteristic X-rays
So, the cause of this radiation is the formation of electron vacancies in the inner shells, due to the penetration of high-energy electrons deep into the atom. The probability that a hard electron will interact increases with the density of the electron clouds. Therefore, collisions are most likely within densely packed inner shells, such as the lowest K-shell. Here the atom is ionized, and a vacancy is formed in the 1s shell.
This vacancy is filled by an electron from the shell with a higher energy, the excess of which is carried away by the X-ray photon. This electron can "fall" from the second shell L, from the third shell M and so on. This is how the characteristic series is formed, in this example, the K-series. An indication of where the electron filling the vacancy comes from is given in the form of a Greek index when designating the series. "Alpha" means that it comes from the L-shell, "beta" - from the M-shell. At present, there is a tendency to replace the Greek letter indices with the Latin ones adopted to designate shells.
The intensity of the alpha line in the series is always the highest, which means that the probability of filling a vacancy from a neighboring shell is the highest.
Now we can answer the question, what is the maximum energy of the characteristic x-ray quantum. It is determined by the difference in the energy values of the levels between which the electron transition occurs, according to the formula E \u003d E n 2 - E n 1, where E n 2 and E n 1 are the energies of the electronic states between which the transition occurred. The highest value of this parameter is given by K-series transitions from the highest possible levels of atoms of heavy elements. But the intensity of these lines (peak heights) is the smallest, since they are the least likely.
If, due to insufficient voltage on the electrodes, a hard electron cannot reach the K-level, it forms a vacancy at the L-level, and a less energetic L-series with longer wavelengths is formed. Subsequent series are born in a similar way.

In addition, when a vacancy is filled, a new vacancy appears in the overlying shell as a result of an electronic transition. This creates the conditions for generating the next series. Electronic vacancies move higher from level to level, and the atom emits a cascade of characteristic spectral series, while remaining ionized.
Fine structure of characteristic spectra
Atomic X-ray spectra of characteristic X-ray radiation are characterized by a fine structure, which is expressed, as in optical spectra, in line splitting.
The fine structure is due to the fact that the energy level - the electron shell - is a set of closely spaced components - subshells. To characterize the subshells, one more, internal quantum number j is introduced, which reflects the interaction of the intrinsic and orbital magnetic moments of the electron.
Due to the influence of the spin-orbit interaction, the energy structure of the atom becomes more complicated, and as a result, the characteristic X-ray radiation has a spectrum that is characterized by split lines with very closely spaced elements.
Fine structure elements are usually denoted by additional digital indices.

The characteristic X-ray radiation has a feature that is reflected only in the fine structure of the spectrum. The transition of an electron to the lowest energy level does not occur from the lower subshell of the overlying level. Such an event has a negligible probability.
The use of X-rays in spectrometry
This radiation, due to its features described by Moseley's law, underlies various X-ray spectral methods for the analysis of substances. When analyzing the X-ray spectrum, either diffraction of radiation by crystals (wave-dispersive method) or detectors sensitive to the energy of absorbed X-ray photons (energy-dispersive method) are used. Most electron microscopes are equipped with some form of X-ray spectrometry attachment.
Wave-dispersive spectrometry is characterized by especially high accuracy. With the help of special filters, the most intense peaks in the spectrum are selected, thanks to which it is possible to obtain almost monochromatic radiation with a precisely known frequency. The anode material is chosen very carefully to ensure that a monochromatic beam of the desired frequency is obtained. Its diffraction on the crystal lattice of the studied substance makes it possible to study the structure of the lattice with great accuracy. This method is also used in the study of DNA and other complex molecules.

One of the features of the characteristic X-ray radiation is also taken into account in gamma spectrometry. This is the high intensity of the characteristic peaks. Gamma spectrometers use lead shielding against external background radiation that interferes with measurements. But lead, absorbing gamma quanta, experiences internal ionization, as a result of which it actively emits in the X-ray range. Additional cadmium shielding is used to absorb the intense peaks of the characteristic x-ray radiation from lead. It, in turn, is ionized and also emits X-rays. To neutralize the characteristic peaks of cadmium, a third shielding layer is used - copper, the X-ray maxima of which lie outside the operating frequency range of the gamma spectrometer.
Spectrometry uses both bremsstrahlung and characteristic X-rays. Thus, in the analysis of substances, the absorption spectra of continuous X-rays by various substances are studied.
1. Bremsstrahlung and characteristic x-rays,
basic properties and characteristics.
In 1895, the German scientist Roentgen first discovered the glow of a fluorescent screen, which was caused by radiation invisible to the eye coming from a portion of the gas discharge tube glass located opposite the cathode. This type of radiation had the ability to pass through substances impenetrable to visible light. Roentgen called them X-rays and established the basic properties that make it possible to use them in various branches of science and technology, including medicine.
X-ray is called radiation with a wavelength of 80-10 -5 nm. Long-wave X-ray radiation overlaps short-wave UV radiation, short-wave overlaps with long-wave g-radiation. In medicine, X-ray radiation with a wavelength of 10 to 0.005 nm is used, which corresponds to a photon energy of 10 2 EV to 0.5 MeV. X-ray radiation is invisible to the eye, therefore, all observations with it are made using fluorescent screens or photographic films, since it causes x-ray luminescence and has a photochemical effect. It is characteristic that the majority of bodies that are impenetrable to optical radiation are largely transparent to X-ray radiation, which has properties common to electromagnetic waves. However, due to the smallness of the wavelength, some properties are difficult to detect. Therefore, the wave nature of radiation was established much later than their discovery.
According to the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic radiation.
Bremsstrahlung X-rays are due to the deceleration of fast moving electrons by the electric field of the atom (nucleus and electrons) of the substance through which they fly. The mechanism of this radiation can be explained by the fact that any moving charge is a current around which a magnetic field is created, the induction (B) of which depends on the speed of the electron. When braking, the magnetic induction decreases and, in accordance with Maxwell's theory, an electromagnetic wave appears.
When electrons decelerate, only part of the energy goes to create an X-ray photon, the other part is spent on heating the anode. The frequency (wavelength) of a photon depends on the initial kinetic energy of the electron and the intensity of its deceleration. Moreover, even if the initial kinetic energy is the same, then the deceleration conditions in the substance will be different, therefore, the emitted photons will have the most diverse energy, and, consequently, the wavelength, i.e. the X-ray spectrum will be continuous. Figure 1 shows the bremsstrahlung spectrum at different voltages U 1
 .
.
If U is expressed in kilovolts and the ratio between other quantities is taken into account, then the formula looks like: l k \u003d 1.24 / U (nm) or l k \u003d 1.24 / U (Å) (1Å \u003d 10 -10 m).
From the graphs above, it can be established that the wavelength l m, which accounts for the maximum radiation energy, is in constant relation to the limiting wavelength l k:
![]() .
.
The wavelength characterizes the energy of a photon, on which the penetrating power of radiation depends when it interacts with matter.
Short-wavelength X-rays usually have a high penetrating power and are called hard, while long-wavelength X-rays are called soft. As can be seen from the above formula, the wavelength at which the maximum radiation energy falls is inversely proportional to the voltage between the anode and cathode of the tube. Increasing the voltage at the anode of the x-ray tube, change the spectral composition of the radiation and increase its hardness.
When the filament voltage changes (the filament temperature of the cathode changes), the number of electrons emitted by the cathode per unit time changes, or, accordingly, the current strength in the tube anode circuit. In this case, the radiation power changes in proportion to the first power of the current. The spectral composition of the radiation will not change.
The total flux (power) of radiation, the distribution of energy over wavelengths, and also the boundary of the spectrum on the side of short wavelengths depend on the following three factors: voltage U, which accelerates electrons and is applied between the anode and cathode of the tube; the number of electrons involved in the formation of radiation, i.e. tube filament current; atomic number Z of the anode material, in which the electron deceleration occurs.
The bremsstrahlung flux is calculated by the formula: , where ![]() ,
,
Z-serial number of an atom of a substance (atomic number).
By increasing the voltage on the x-ray tube, one can notice the appearance of separate lines (line spectrum) against the background of continuous bremsstrahlung radiation, which corresponds to the characteristic x-ray radiation. It arises during the transition of electrons between the inner shells of atoms in a substance (shells K, L, M). The line character of the characteristic radiation spectrum arises due to the fact that accelerated electrons penetrate deep into the atoms and knock out electrons from their inner layers outside the atom. Electrons (Fig. 2) from the upper layers pass to free places, as a result of which X-ray photons are emitted with a frequency corresponding to the difference in the transition energy levels. The lines in the spectrum of characteristic radiation are combined into series corresponding to transitions of electrons with a higher level at the level of K, L, M.
The external action, as a result of which the electron is knocked out of the inner layers, must be strong enough. In contrast to optical spectra, the characteristic x-ray spectra of different atoms are of the same type. The uniformity of these spectra is due to the fact that the inner layers of different atoms are the same and differ only energetically, because the force effect from the side of the nucleus increases as the ordinal number of the element increases. This leads to the fact that the characteristic spectra shift towards higher frequencies with increasing nuclear charge. This relationship is known as Moseley's law: ![]() , where A and B are constants; Z-order number of the element.
, where A and B are constants; Z-order number of the element.
There is another difference between X-ray and optical spectra. The characteristic spectrum of an atom does not depend on the chemical compound in which the atom is included. So, for example, the X-ray spectrum of the oxygen atom is the same for O, O 2 , H 2 O, while the optical spectra of these compounds are significantly different. This feature of the x-ray spectra of atoms served as the basis for the name "characteristic".
Characteristic radiation occurs whenever there are free places in the inner layers of an atom, regardless of the reasons that caused it. For example, it accompanies one of the types of radioactive decay, which consists in the capture of an electron from the inner layer by the nucleus.
2. The device of x-ray tubes and protozoa
x-ray machine.
The most common source of X-ray radiation is an X-ray tube - a two-electrode vacuum device (Fig. 3). It is a glass container (p = 10 -6 - 10 -7 mm Hg) with two electrodes - anode A and cathode K, between which a high voltage is created. The heated cathode (K) emits electrons. Anode A is often referred to as the anticathode. It has an inclined surface in order to direct the resulting X-ray radiation at an angle to the axis of the tube. The anode is made of a metal with good thermal conductivity (copper) to remove the heat generated by the impact of electrons. At the beveled end of the anode there is a plate Z made of refractory metal (tungsten) with a high atomic number, called the anode mirror. In some cases, the anode is specially cooled with water or oil. For diagnostic tubes, the pinpointness of the X-ray source is important, which can be achieved by focusing the electrons in one place of the anode. Therefore, constructively, two opposite tasks have to be taken into account: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different parts of the anode. For this reason, some X-ray tubes are manufactured with a rotating anode.
In a tube of any design, electrons accelerated by the voltage between the anode and the cathode fall on the anode mirror and penetrate deep into the substance, interact with atoms and are decelerated by the field of atoms. This produces bremsstrahlung X-rays. Simultaneously with the bremsstrahlung, a small amount (several percent) of characteristic radiation is formed. Only 1-2% of the electrons that hit the anode cause bremsstrahlung, and the rest cause a thermal effect. For the concentration of electrons, the cathode has a guide cap. The part of the tungsten mirror on which the main electron flow falls is called the focus of the tube. The width of the radiation beam depends on its area (focus sharpness).
To power the tube, two sources are required: a high voltage source for the anode circuit and a low voltage source (6-8 V) to power the filament circuit. Both sources must be independently regulated. By changing the anode voltage, the hardness of the X-ray radiation is regulated, and by changing the incandescence, the current of the output circuit and, accordingly, the radiation power.
Schematic diagram of the simplest X-ray machine is shown in Fig.4. The circuit has two high voltage transformers Tr.1 and Tr.2 for powering the filament. The high voltage on the tube is regulated by an autotransformer Tr.3 connected to the primary winding of the transformer Tr.1. Switch K regulates the number of turns of the autotransformer winding. In this regard, the voltage of the secondary winding of the transformer, supplied to the anode of the tube, also changes, i.e. hardness is adjustable.
The filament current of the tube is regulated by a rheostat R, included in the primary circuit of the transformer Tr.2. The anode circuit current is measured with a milliammeter. The voltage applied to the electrodes of the tube is measured with a kV kilovoltmeter, or the voltage in the anode circuit can be judged by the position of switch K. The filament current, regulated by the rheostat, is measured with an ammeter A. In the scheme under consideration, the x-ray tube simultaneously rectifies a high alternating voltage.
It is easy to see that such a tube radiates only in one half-cycle of alternating current. Therefore, its power will be small. In order to increase the radiated power, many devices use high-voltage full-wave X-ray rectifiers. For this purpose, 4 special kenotrons are used, which are connected in a bridge circuit. An x-ray tube is included in one diagonal of the bridge.
3. Interaction of X-ray radiation with matter
(coherent scattering, incoherent scattering, photoelectric effect).
When X-rays fall on a body, it is reflected from it in a small amount, but mostly passes deep into. In the mass of the body, radiation is partially absorbed, partially scattered, and partially passes through. Passing through the body, X-ray photons interact mainly with the electrons of the atoms and molecules of the substance. Registration and use of X-ray radiation, as well as its impact on biological objects, is determined by the primary processes of interaction of an X-ray photon with electrons. Three main processes take place depending on the ratio of photon energy E and ionization energy AI.
A) coherent scattering.
Scattering of long-wavelength X-rays occurs mainly without changing the wavelength, and it is called coherent. The interaction of a photon with the electrons of the inner shells, tightly bound to the nucleus, only changes its direction, without changing its energy, and hence the wavelength (Fig. 5).
Coherent scattering occurs if the photon energy is less than the ionization energy: E = hn<А И. Так как энергия фотона и энергия атома не изменяется, то когерентное рассеяние не вызывает биологического действия. Однако при создании защиты от рентгеновского излучения следует учитывать возможность изменения направления первичного пучка.
b) Incoherent scattering (Compton effect).
In 1922, A. Compton, observing the scattering of hard X-rays, discovered a decrease in the penetrating power of the scattered beam compared to the incident beam. The scattering of X-rays with changing wavelength is called the Compton effect. It occurs when a photon of any energy interacts with the electrons of the outer shells of atoms weakly bound to the nucleus (Fig. 6). An electron is detached from an atom (such electrons are called recoil electrons). The energy of the photon decreases (the wavelength increases accordingly), and the direction of its movement also changes. The Compton effect occurs if the X-ray photon energy is greater than the ionization energy: , . In this case, recoil electrons with kinetic energy E K appear. Atoms and molecules become ions. If E K is significant, then electrons can ionize neighboring atoms by collision, forming new (secondary) electrons.
V) Photoelectric effect.
If the energy of a photon hn is sufficient to detach an electron, then when interacting with an atom, the photon is absorbed, and the electron is detached from it. This phenomenon is called the photoelectric effect. The atom is ionized (photoinization). In this case, the electron acquires kinetic energy and, if the latter  is significant, then it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances, this leads to the subsequent emission of photons in the visible radiation region (X-ray luminescence), and in tissues, to the activation of molecules and photochemical reactions.
is significant, then it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances, this leads to the subsequent emission of photons in the visible radiation region (X-ray luminescence), and in tissues, to the activation of molecules and photochemical reactions.
The photoelectric effect is typical for photons with an energy of the order of 0.5-1 MeV.
The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. When X-ray radiation enters a substance, a number of processes can occur before the energy of an X-ray photon is converted into the energy of thermal motion.
As a result of the above processes, the primary X-ray flux is weakened. This process obeys Bouguer's law. We write it in the form: Ф =Ф 0 e - mx, where m is a linear attenuation coefficient that depends on the nature of the substance (mainly on density and atomic number) and on the radiation wavelength (photon energy). It can be represented as consisting of three terms corresponding to coherent scattering, incoherent scattering, and the photoelectric effect: ![]() .
.
Since the linear absorption coefficient depends on the density of the substance, it is preferable to use the mass attenuation coefficient, which is equal to the ratio of the linear attenuation coefficient to the density of the absorber and does not depend on the density of the substance. The dependence of the X-ray flux (intensity) on the thickness of the absorbing filter is shown in Fig. 7 for H 2 O, Al, and Cu. Calculations show that a layer of water 36 mm thick, aluminum 15 mm and copper 1.6 mm reduce the X-ray intensity by 2 times. This thickness is called the half layer thickness d. If a substance attenuates X-ray radiation by half, then ![]() , Then
, Then ![]() , or ,
, or , ![]() ; ; . Knowing the thickness of the half layer, you can always determine m. Dimension .
; ; . Knowing the thickness of the half layer, you can always determine m. Dimension .
4. The use of x-rays in medicine
(fluoroscopy, radiography, X-ray tomography, fluorography, radiotherapy).
One of the most common applications of X-rays in medicine is the transillumination of internal organs for diagnostic purposes - X-ray diagnostics.
For diagnostics, photons with an energy of 60-120 keV are used. In this case, the mass absorption coefficient is determined mainly by the photoelectric effect. Its value is proportional to l 3 (in which the large penetrating power of hard radiation is manifested) and proportional to the third power of the number of atoms of the substance - absorber: , where K is the coefficient of proportionality.
The human body consists of tissues and organs that have different absorbing capacity in relation to X-rays. Therefore, when it is illuminated with X-rays, a non-uniform shadow image is obtained on the screen, which gives a picture of the location of internal organs and tissues. The densest radiation-absorbing tissues (heart, large vessels, bones) are seen as dark, while the less absorbing tissues (lungs) are seen as light.
In many cases, it is possible to judge their normal or pathological state. X-ray diagnostics uses two main methods: fluoroscopy (transmission) and radiography (image). If the organ under study and the tissues surrounding it approximately equally absorb the X-ray flux, then special contrast agents are used. So, for example, on the eve of an X-ray examination of the stomach or intestines, a mushy mass of barium sulfate is given, in which case one can see their shadow image. In fluoroscopy and radiography, an x-ray image is a summary image of the entire thickness of the object through which the x-rays pass. The most clearly defined are those details that are closer to the screen or film, and the distant ones become fuzzy and blurry. If in some organ there is a pathologically altered area, for example, the destruction of lung tissue inside an extensive focus of inflammation, then in some cases this area on the x-ray in the amount of shadows can be “lost”. To make it visible, a special method is used - tomography (layered recording), which allows you to take pictures of individual layers of the area under study. This kind of layer-by-layer tomograms is obtained using a special apparatus called a tomograph, in which the X-ray tube (RT) and film (Fp) are periodically, jointly, in antiphase moved relative to the study area. In this case, X-rays at any position of the RT will pass through the same point of the object (changed area), which is the center relative to which the RT and FP periodically move. The shadow image of the area will be captured on film. By changing the position of the “swing center”, it is possible to obtain layered images of the object. Using a thin beam of X-rays, a special screen (instead of Fp) consisting of semiconductor detectors of ionizing radiation, it is possible to process the image during tomography using a computer. This modern variant of tomography is called computed tomography. Tomography is widely used in the study of the lungs, kidneys, gallbladder, stomach, bones, etc.
The brightness of the image on the screen and the exposure time on the film depends on the intensity of the X-ray radiation. When using it for diagnostics, the intensity cannot be high, so as not to cause an undesirable biological effect. Therefore, there are a number of technical devices that improve the brightness of the image at low X-ray intensities. One of these devices is an image intensifier tube.
Another example is fluorography, in which an image is obtained on a sensitive small-format film from a large X-ray luminescent screen. When shooting, a lens of large aperture is used, the finished pictures are examined on a special magnifier.
Fluorography combines a great ability to detect latent diseases (diseases of the chest, gastrointestinal tract, paranasal sinuses, etc.) with a significant throughput, and therefore is a very effective method of mass (in-line) research.
Since photographing an x-ray image during fluorography is performed using photographic optics, the image on the fluorogram is reduced compared to the x-ray. In this regard, the resolution of the fluorogram (i.e., the visibility of small details) is less than that of a conventional radiograph, however, it is greater than with fluoroscopy.
A device was designed - a tomofluorograph, which makes it possible to obtain fluorograms of body parts and individual organs at a given depth - the so-called layered images (sections) - tomofluorograms.
X-ray radiation is also used for therapeutic purposes (X-ray therapy). The biological effect of radiation is to disrupt the vital activity of cells, especially rapidly developing ones. In this regard, X-ray therapy is used to influence malignant tumors. It is possible to choose a dose of radiation sufficient for the complete destruction of the tumor with relatively minor damage to the surrounding healthy tissues, which are restored due to subsequent regeneration.
Intensity- quantitative characteristic of x-ray radiation, which is expressed by the number of rays emitted by the tube per unit time. The intensity of X-rays is measured in milliamps. Comparing it with the intensity of visible light from a conventional incandescent lamp, we can draw an analogy: for example, a 20-watt lamp will shine with one intensity, or power, and a 200-watt lamp with another, while the quality of the light itself (its spectrum) is the same. The intensity of X-ray radiation is, in fact, its quantity. Each electron creates one or more radiation quanta on the anode, therefore, the number of X-rays during exposure of the object is regulated by changing the number of electrons tending to the anode and the number of interactions of electrons with atoms of the tungsten target, which can be done in two ways:
1. By changing the degree of incandescence of the cathode spiral using a step-down transformer (the number of electrons generated during emission will depend on how hot the tungsten spiral is, and the number of radiation quanta will depend on the number of electrons);
2. By changing the value of the high voltage supplied by the step-up transformer to the poles of the tube - the cathode and the anode (the higher the voltage is applied to the poles of the tube, the more kinetic energy the electrons receive, which, due to their energy, can interact with several atoms of the anode substance in turn - see. rice. 5; electrons with low energy will be able to enter into a smaller number of interactions).
The X-ray intensity (anode current) multiplied by the exposure (tube time) corresponds to the X-ray exposure, which is measured in mAs (milliamps per second). Exposure is a parameter that, like intensity, characterizes the amount of rays emitted by an x-ray tube. The only difference is that the exposure also takes into account the operating time of the tube (for example, if the tube works for 0.01 sec, then the number of rays will be one, and if it is 0.02 sec, then the number of rays will be different - twice as many). The radiation exposure is set by the radiologist on the control panel of the X-ray machine, depending on the type of examination, the size of the object under study and the diagnostic task.
Rigidity- qualitative characteristic of x-ray radiation. It is measured by the high voltage on the tube - in kilovolts. Determines the penetrating power of x-rays. It is regulated by the high voltage supplied to the X-ray tube by a step-up transformer. The higher the potential difference is created on the electrodes of the tube, the more force the electrons repel from the cathode and rush to the anode, and the stronger their collision with the anode. The stronger their collision, the shorter the wavelength of the resulting X-ray radiation and the higher the penetrating power of this wave (or the hardness of the radiation, which, like the intensity, is regulated on the control panel by the voltage parameter on the tube - kilovoltage).
Rice. 7 - Dependence of the wavelength on the energy of the wave:
λ - wavelength;
E - wave energy
· The higher the kinetic energy of moving electrons, the stronger their impact on the anode and the shorter the wavelength of the resulting X-ray radiation. X-ray radiation with a long wavelength and low penetrating power is called "soft", with a short wavelength and high penetrating power - "hard".
 Rice. 8 - The ratio of the voltage on the X-ray tube and the wavelength of the resulting X-ray radiation:
Rice. 8 - The ratio of the voltage on the X-ray tube and the wavelength of the resulting X-ray radiation:
· The higher the voltage is applied to the poles of the tube, the stronger the potential difference appears on them, therefore, the kinetic energy of moving electrons will be higher. The voltage on the tube determines the speed of the electrons and the force of their collision with the anode material, therefore, the voltage determines the wavelength of the resulting X-ray radiation.



