Alkenes are chemically active. Their chemical properties are largely determined by the presence of a double bond. For alkenes, electrophilic addition reactions and radical addition reactions are most characteristic. Nucleophilic addition reactions usually require a strong nucleophile and are not typical of alkenes. Alkenes easily enter into reactions of oxidation, addition, and are also capable of allyl radical substitution.
Addition reactions
Hydrogenation Hydrogen addition (hydrogenation reaction) to alkenes is carried out in the presence of catalysts. Most often, crushed metals are used - platinum, nickel, palladium, etc. As a result, the corresponding alkanes (saturated hydrocarbons) are formed.
$CH_2=CH_2 + H2 → CH_3–CH_3$
addition of halogens. Alkenes easily react with chlorine and bromine under normal conditions to form the corresponding dihaloalkanes, in which the halogen atoms are located at neighboring carbon atoms.
Remark 1
When alkenes interact with bromine, the yellow-brown color of bromine is discolored. This is one of the oldest and simplest qualitative reactions for unsaturated hydrocarbons, since alkynes and alkadienes also react similarly.
$CH_2=CH_2 + Br_2 → CH_2Br–CH_2Br$
addition of hydrogen halides. When ethylene hydrocarbons react with hydrogen halides ($HCl$, $HBr$), haloalkanes are formed, the direction of the reaction depends on the structure of alkenes.
In the case of ethylene or symmetrical alkenes, the addition reaction occurs unambiguously and leads to the formation of only one product:
$CH_2=CH_2 + HBr → CH_3–CH_2Br$
In the case of unsymmetrical alkenes, the formation of two different addition reaction products is possible:
Remark 2
In fact, basically only one reaction product is formed. The regularity of the direction of passage of such reactions was established by the Russian chemist V.V. Markovnikov in 1869 It is called Markovnikov's rule. In the interaction of hydrogen halides with unsymmetrical alkenes, the hydrogen atom joins at the place where the double bond is broken in the most hydrogenated carbon atom, that is, before it is connected to a large number of hydrogen atoms.
Markovnikov formulated this rule on the basis of experimental data, and only much later did it receive a theoretical justification. Consider the reaction of propylene with hydrogen chloride.
One of the features of the $p$ bond is its ability to be easily polarized. Under the influence of the methyl group (positive inductive effect + $I$) in the propene molecule, the electron density of the $p$ bond is shifted to one of the carbon atoms (= $CH_2$). As a result, a partial negative charge ($\delta -$) appears on it. On the other carbon atom of the double bond, a partial positive charge arises ($\delta +$).
This distribution of electron density in the propylene molecule determines the location of the future attack by the proton. This is the carbon atom of the methylene group (= $CH_2$), which carries a partial negative charge $\delta-$. And chlorine, accordingly, attacks the carbon atom with a partial positive charge $\delta+$.
As a consequence, the main reaction product of propylene with hydrogen chloride is 2-chloropropane.
Hydration
Hydration of alkenes occurs in the presence of mineral acids and obeys the Markovnikov rule. The reaction products are alcohols
$CH_2=CH_2 + H_2O → CH_3–CH_2–OH$
Alkylation
The addition of alkanes to alkenes in the presence of an acid catalyst ($HF$ or $H_2SO_4$) at low temperatures leads to the formation of hydrocarbons with a higher molecular weight and is often used in industry to produce motor fuel
$R–CH_2=CH_2 + R’–H → R–CH_2–CH_2–R’$
Oxidation reactions
The oxidation of alkenes can occur, depending on the conditions and types of oxidizing reagents, both with the breaking of the double bond and with the preservation of the carbon skeleton:
polymerization reactions
Alkene molecules are capable of adding to each other under certain conditions with the opening of $\pi$-bonds and the formation of dimers, trimers or high-molecular compounds - polymers. The polymerization of alkenes can proceed both by free radical and cation-anion mechanisms. Acids, peroxides, metals, etc. are used as polymerization initiators. The polymerization reaction is also carried out under the influence of temperature, irradiation, and pressure. A typical example is the polymerization of ethylene to form polyethylene
$nCH_2=CH_2 → (–CH_2–CH_(2^–))_n$
Substitution reactions
Substitution reactions for alkenes are not typical. However, at high temperatures (above 400 °C), radical addition reactions, which are reversible, are suppressed. In this case, it becomes possible to carry out the substitution of the hydrogen atom in the allyl position while maintaining the double bond
$CH_2=CH–CH_3 + Cl_2 – CH_2=CH–CH_2Cl + HCl$
Knowledge Hypermarket >>Chemistry >>Chemistry Grade 10 >> Chemistry: Alkenes
Unsaturated hydrocarbons include hydrocarbons containing multiple bonds between carbon atoms in molecules. Unsaturated are alkenes, alkynes, alkadienes (polyenes). Cyclic hydrocarbons containing a double bond in the cycle (cycloalkenes), as well as cycloalkanes with a small number of carbon atoms in the cycle (three or four atoms) also have an unsaturated character. The property of "unsaturation" is associated with the ability of these substances to enter into addition reactions, primarily hydrogen, with the formation of saturated, or saturated, hydrocarbons - alkanes.
Structure
Alkenes - acyclic, containing in the molecule, in addition to single bonds, one double bond between carbon atoms and corresponding to the general formula C n H 2n.
Alkenes received their second name - "olefins" by analogy with unsaturated fatty acids (oleic, linoleic), the remains of which are part of liquid fats - oils (from the English oil - oil).
Carbon atoms between which there is a double bond, as you know, are in a state of sp 2 hybridization. This means that one s- and two p-orbitals participate in hybridization, while one p-orbital remains unhybridized. The overlap of hybrid orbitals leads to the formation of an α-bond, and due to the unhybridized α-orbitals of neighboring ethylene molecules, carbon atoms form a second, P-connection. Thus, a double bond consists of one z- and one p-bond.
The hybrid orbitals of the atoms that form the double bond are in the same plane, while the orbitals that form the n-bond are perpendicular to the plane of the molecule (see Fig. 5).
A double bond (0.132 nm) is shorter than a single bond, and its energy is greater, that is, it is more durable. Nevertheless, the presence of a mobile, easily polarizable 7r bond leads to the fact that alkenes are chemically more active than alkanes and are able to enter into addition reactions.
Homologous series of ethene
Unbranched alkenes make up the homologous series of ethene (ethylene).
C2H4 - ethene, C3H6 - propene, C4H8 - butene, C5H10 - pentene, C6H12 - hexene, etc.
Isomerism and nomenclature
For alkenes, as well as for alkanes, structural isomerism is characteristic. Structural isomers, as you remember, differ from each other in the structure of the carbon skeleton. The simplest alkene, which is characterized by structural isomers, is butene.
CH3-CH2-CH=CH2 CH3-C=CH2
l
CH3
butene-1 methylpropene
A special type of structural isomerism is the double bond position isomerism:
CH3-CH2-CH=CH2 CH3-CH=CH-CH3
butene-1 butene-2
Almost free rotation of carbon atoms is possible around a single carbon-carbon bond, so alkane molecules can take on a wide variety of shapes. Rotation around the double bond is impossible, which leads to the appearance of another type of isomerism in alkenes - geometric, or cis-trans isomerism.
Cis-isomers differ from thorax-isomers by the spatial arrangement of molecular fragments (in this case, methyl groups) relative to the plane P relationships, and hence properties.
Alkenes are isomeric to cycloalkanes (interclass isomerism), for example:
ch2=ch-ch2-ch2-ch2-ch3
hexene-1 cyclohexane
Nomenclature alkenes, developed by IUPAC, is similar to the nomenclature of alkanes.
1. Main circuit selection
The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in a molecule. In the case of alkenes, the main chain must contain a double bond.
2. Numbering of atoms of the main chain
The numbering of the atoms of the main chain starts from the end to which the double bond is closest. For example, the correct connection name is
ch3-chn-ch2-ch=ch-ch3 ch3
5-methylhexene-2, not 2-methylhexene-4 as might be expected.
If it is impossible to determine the beginning of the numbering of atoms in the chain by the location of the double bond, then it is determined by the position of the substituents in the same way as for saturated hydrocarbons.
CH3-CH2-CH=CH-CH-CH3
l
CH3
2-methylhexene-3
3. Name formation
The names of alkenes are formed in the same way as the names of alkanes. At the end of the name, the number of the carbon atom at which the double bond begins is indicated, and the suffix, indicating that the compound belongs to the class of alkenes, -ene.
Receipt
1. Cracking of petroleum products. In the process of thermal cracking of saturated hydrocarbons, along with the formation of alkanes, the formation of alkenes occurs.
2. Dehydrogenation of saturated hydrocarbons. When alkanes are passed over a catalyst at a high temperature (400-600 °C), a hydrogen molecule is split off and an alkene is formed: 
3. Dehydration of alcohols (cleavage of water). The effect of water-removing agents (H2804, Al203) on monohydric alcohols at high temperatures leads to the elimination of a water molecule and the formation of a double bond:
This reaction is called intramolecular dehydration (in contrast to intermolecular dehydration, which leads to the formation of ethers and will be studied in § 16 "Alcohols").
4. Dehydrohalogenation (elimination of hydrogen halide).
When a haloalkane reacts with an alkali in an alcoholic solution, a double bond is formed as a result of the elimination of a hydrogen halide molecule.
Note that this reaction produces predominantly butene-2 rather than butene-1, corresponding to Zaitsev's rule:
When a hydrogen halide is split off from secondary and tertiary haloalkanes, a hydrogen atom is split off from the least hydrogenated carbon atom.
5. Dehalogenation. Under the action of zinc on a dibromo derivative of an alkane, halogen atoms are split off from neighboring carbon atoms and a double bond is formed: 
Physical Properties
The first three representatives of the homologous series of alkenes are gases, substances of the composition C5H10-C16H32 are liquids, higher alkenes are solids.
The boiling and melting points naturally increase with an increase in the molecular weight of the compounds.
Chemical properties
Addition reactions
Recall that a distinctive feature of the representatives of unsaturated hydrocarbons - alkenes is the ability to enter into addition reactions. Most of these reactions proceed by the mechanism of electrophilic addition.
1. Hydrogenation of alkenes. Alkenes are able to add hydrogen in the presence of hydrogenation catalysts - metals - platinum, palladium, nickel:
CH3-CH2-CH=CH2 + H2 -> CH3-CH2-CH2-CH3
This reaction proceeds both at atmospheric and elevated pressure and does not require high temperature, as it is exothermic. With an increase in temperature on the same catalysts, the reverse reaction, dehydrogenation, can occur.
2. Halogenation (addition of halogens). The interaction of an alkene with bromine water or a solution of bromine in an organic solvent (СCl4) leads to a rapid discoloration of these solutions as a result of the addition of a halogen molecule to the alkene and the formation of dihaloalkanes.
Markovnikov Vladimir Vasilievich
(1837-1904)
Russian organic chemist. Formulated (1869) rules on the direction of reactions of substitution, elimination, double bond addition and isomerization, depending on the chemical structure. Investigated (since 1880) the composition of oil, laid the foundations of petrochemistry as an independent science. Opened (1883) a new class of organic substances - cyclo-paraffins (naphthenes).
3. Hydrohalogenation (addition of hydrogen halide).
The hydrogen halide addition reaction will be discussed in more detail below. This reaction obeys Markovnikov's rule:
When a hydrogen halide is added to an alkene, hydrogen is attached to a more hydrogenated carbon atom, i.e., an atom at which there are more hydrogen atoms, and a halogen to a less hydrogenated one.
4. Hydration (water addition). Hydration of alkenes leads to the formation of alcohols. For example, the addition of water to ethene underlies one of the industrial methods for producing ethyl alcohol:
CH2=CH2 + H2O -> CH3-CH2OH
ethene ethanol
Note that a primary alcohol (with a hydroxyl group at the primary carbon) is formed only when ethene is hydrated. When propene or other alkenes are hydrated, secondary alcohols are formed. 
This reaction also proceeds in accordance with Markovnikov's rule - the hydrogen cation is added to the more hydrogenated carbon atom, and the hydroxy group is added to the less hydrogenated one.
5. Polymerization. A special case of addition is the polymerization reaction of alkenes:
This addition reaction proceeds by a free radical mechanism.
Oxidation reactions
Like any organic compounds, alkenes burn in oxygen to form CO2 and H20.
Unlike alkanes, which are resistant to oxidation in solutions, alkenes are easily oxidized by aqueous solutions of potassium permanganate. In neutral or slightly alkaline solutions, alkenes are oxidized to diols (dihydric alcohols), and hydroxyl groups are attached to those atoms between which a double bond existed before oxidation.
As you already know, unsaturated hydrocarbons - alkenes are able to enter into addition reactions. Most of these reactions proceed by the mechanism of electrophilic addition.
electrophilic addition
Electrophilic reactions are reactions that occur under the action of electrophiles - particles that have a lack of electron density, such as an unfilled orbital. The simplest electrophilic particle is the hydrogen cation. It is known that the hydrogen atom has one electron per 3-in-orbital. A hydrogen cation is formed when an atom loses that electron, so the hydrogen cation has no electrons at all:
H - 1e - -> H +
In this case, the cation has a rather high electron affinity. The combination of these factors makes the hydrogen cation a fairly strong electrophilic particle.
The formation of a hydrogen cation is possible during the electrolytic dissociation of acids:
HBr -> H + + Br -
It is for this reason that many electrophilic reactions occur in the presence and with the participation of acids.
Electrophilic particles, as mentioned earlier, act on systems containing regions of increased electron density. An example of such a system can be a multiple (double or triple) carbon-carbon bond.
You already know that the carbon atoms between which a double bond is formed are in a state of sp 2 hybridization. Unhybridized p-orbitals of neighboring carbon atoms, which are in the same plane, overlap, forming P-bond, which is less strong than the z-bond, and, most importantly, is easily polarized under the action of an external electric field. This means that when a positively charged particle approaches, the electrons of the TC bond are displaced in its direction and the so-called P- complex.
It turns out P-complex and upon addition of a hydrogen cation to P-connections. The hydrogen cation, as it were, stumbles upon an electron density protruding from the plane of the molecule P-links and joins it. ![]()
At the next stage, the complete displacement of the electron pair occurs. P-bonds to one of the carbon atoms, which leads to the appearance of a lone pair of electrons on it. The orbital of the carbon atom on which this pair is located and the unfilled orbital of the hydrogen cation overlap, which leads to the formation of a covalent bond by the donor-acceptor mechanism. At the same time, the second carbon atom remains an unfilled orbital, i.e., a positive charge.
The resulting particle is called a carbocation because it contains a positive charge on the carbon atom. This particle can combine with any anion, a particle that has an unshared electron pair, i.e., a nucleophile.
Consider the mechanism of the electrophilic addition reaction using the example of hydrobromination (addition of hydrogen bromide) of ethene:
CH2= CH2 + HBr --> CHBr-CH3
The reaction begins with the formation of an electrophilic particle - a hydrogen cation, which occurs as a result of the dissociation of a hydrogen bromide molecule.
Hydrogen cation attacks P-connection, forming P-a complex that quickly converts to a carbocation: 
Now consider a more complicated case.
The addition reaction of hydrogen bromide to ethene proceeds unambiguously, and the interaction of hydrogen bromide with propene can theoretically give two products: 1-bromopropane and 2-bromopropane. Experimental data show that mainly 2-bromopropane is obtained.
In order to explain this, we will have to consider an intermediate particle - a carbocation.
The addition of a hydrogen cation to propene can lead to the formation of two carbocations: if the hydrogen cation is attached to the first carbon atom, to the atom that is at the end of the chain, then the second one, i.e., in the center of the molecule (1), will have a positive charge; if it joins the second, then the first atom (2) will have a positive charge. 
The preferred direction of the reaction will depend on which carbocation will be more in the reaction medium, which, in turn, is determined by the stability of the carbocation. The experiment shows the predominant formation of 2-bromopropane. This means that the formation of carbocation (1) with a positive charge on the central atom occurs to a greater extent.
The greater stability of this carbocation is explained by the fact that the positive charge on the central carbon atom is compensated by the positive inductive effect of two methyl groups, the total effect of which is higher than the +/- effect of one ethyl group:
The patterns of reactions of hydrohalogenation of alkenes were studied by the famous Russian chemist V. V. Markovnikov, a student of A. M. Butlerov, who, as mentioned above, formulated the rule that bears his name.
This rule was established empirically, that is, empirically. At present, we can give a completely convincing explanation of it.
Interestingly, other electrophilic addition reactions also obey the Markovnikov rule, so it would be correct to formulate it in a more general form.
In electrophilic addition reactions, an electrophile (a particle with an unfilled orbital) is attached to a more hydrogenated carbon atom, and a nucleophile (a particle with a lone pair of electrons) is attached to a less hydrogenated one.
Polymerization
A special case of the addition reaction is the polymerization of alkenes and their derivatives. This reaction proceeds by the mechanism of free radical addition:
Polymerization is carried out in the presence of initiators - peroxide compounds, which are a source of free radicals. Peroxide compounds are called substances, the molecules of which include the -O-O- group. The simplest peroxide compound is hydrogen peroxide HOOH.
At a temperature of 100 °C and a pressure of 100 MPa, homolysis of an unstable oxygen-oxygen bond occurs and the formation of radicals - polymerization initiators. Under the action of KO- radicals, polymerization is initiated, which develops as a free radical addition reaction. Chain growth stops when the reaction mixture is recombined radicals - polymer chain and radicals or KOCH2CH2-.
Using the reaction of free radical polymerization of substances containing a double bond, a large number of macromolecular compounds are obtained: 
The use of alkenes with various substituents makes it possible to synthesize a wide range of polymeric materials with a wide range of properties. 
All these polymeric compounds are widely used in various fields of human activity - industry, medicine, are used to manufacture equipment for biochemical laboratories, some are intermediates for the synthesis of other macromolecular compounds.
Oxidation
You already know that in neutral or slightly alkaline solutions, alkenes are oxidized to diols (dihydric alcohols). In an acidic environment (a solution acidified with sulfuric acid), the double bond is completely destroyed and the carbon atoms between which the double bond existed are converted into carbon atoms of the carboxyl group: 
Destructive oxidation of alkenes can be used to determine their structure. So, for example, if acetic and propionic acids are obtained during the oxidation of some alkene, this means that pentene-2 has undergone oxidation, and if butyric (butanoic) acid and carbon dioxide are obtained, then the initial hydrocarbon is pentene-1.
Application
Alkenes are widely used in the chemical industry as a raw material for the production of various organic substances and materials.
So, for example, ethene is the starting material for the production of ethanol, ethylene glycol, epoxides, dichloroethane.
A large amount of ethene is processed into polyethylene, which is used for the manufacture of packaging films, dishes, pipes, and electrical insulating materials.
Glycerin, acetone, isopropanol, solvents are obtained from propene. Polymerization of propene produces polypropylene, which is superior to polyethylene in many respects: it has a higher melting point and chemical resistance.
At present, fibers with unique properties are produced from polymers - analogues of polyethylene. For example, polypropylene fiber is stronger than all known synthetic fibers.
Materials made from these fibers are promising and are increasingly used in various fields of human activity.

1. What types of isomerism are characteristic of alkenes? Write the formulas for the possible isomers of pentene-1.
2. What compounds can be obtained from: a) isobutene (2-methylpropene); b) butene-2; c) butene-1? Write the equations for the corresponding reactions.
3. Decipher the following chain of transformations. Name compounds A, B, C. 4. Suggest a method for obtaining 2-chloropropane from 1-chloro-propane. Write the equations for the corresponding reactions.
5. Suggest a method for purifying ethane from ethylene impurities. Write the equations for the corresponding reactions.
6. Give examples of reactions that can be used to distinguish between saturated and unsaturated hydrocarbons.
7. Complete hydrogenation of 2.8 g of alkene consumed 0.896 l of hydrogen (n.a.). What is the molecular weight and structural formula of this compound, which has a normal chain of carbon atoms?
8. What gas is in the cylinder (ethene or propene), if it is known that it took 90 cm3 (n.a.) of oxygen to completely burn 20 cm3 of this gas?
9*. When an alkene reacts with chlorine in the dark, 25.4 g of dichloride is formed, and when this alkene of the same mass reacts with bromine in carbon tetrachloride, 43.2 g of dibromide is formed. Set all possible structural formulas of the starting alkene.
Discovery history
From the above material, we have already understood that ethylene is the ancestor of the homologous series of unsaturated hydrocarbons, which has one double bond. Their formula is C n H 2n and they are called alkenes.
The German physician and chemist Becher in 1669 was the first to obtain ethylene by the action of sulfuric acid on ethyl alcohol. Becher found that ethylene is more reactive than methane. But, unfortunately, at that time, the scientist could not identify the gas received, therefore he did not assign any name to it.
A little later, Dutch chemists also used the same method for obtaining ethylene. And since, when interacting with chlorine, it had the property of forming an oily liquid, it accordingly received the name "oxygen gas". Later it became known that this liquid is dichloroethane.
In French, the term "oily" sounds like oléfiant. And after other hydrocarbons of this type were discovered, Antoine Fourcroix, a French chemist and scientist, introduced a new term that became common to the entire class of olefins or alkenes.
But already at the beginning of the nineteenth century, the French chemist J. Gay-Lussac showed that ethanol consists not only of "oily" gas, but also of water. In addition, the same gas was found in ethyl chloride.
And although chemists determined that ethylene consists of hydrogen and carbon, and already knew the composition of substances, they could not find its real formula for a long time. And only in 1862, E. Erlenmeyer managed to prove the presence of a double bond in the ethylene molecule. This was also recognized by the Russian scientist A. M. Butlerov and confirmed the correctness of this point of view experimentally.
Finding in nature and the physiological role of alkenes
Many are interested in the question of where alkenes can be found in nature. So, it turns out that they practically do not occur in nature, since its simplest representative, ethylene, is a hormone for plants and is synthesized in them only in small quantities.
True, in nature there is such an alkene as muscalur. This one of the natural alkenes is a sexual attractant of the female house fly.
It is worth paying attention to the fact that, having a high concentration, lower alkenes have a narcotic effect that can cause convulsions and irritation of the mucous membranes.
Application of alkenes
The life of modern society today is difficult to imagine without the use of polymeric materials. Since, unlike natural materials, polymers have different properties, they are easy to process, and if you look at the price, they are relatively cheap. Another important aspect in favor of polymers is that many of them can be recycled.
Alkenes have found their application in the production of plastics, rubbers, films, Teflon, ethyl alcohol, acetaldehyde and other organic compounds.

In agriculture, it is used as a means that accelerates the process of fruit ripening. Propylene and butylenes are used to produce various polymers and alcohols. But in the production of synthetic rubber, isobutylene is used. Therefore, we can conclude that alkenes cannot be dispensed with, since they are the most important chemical raw materials.
Industrial use of ethylene
On an industrial scale, propylene is usually used for the synthesis of polypropylene and for the production of isopropanol, glycerol, butyric aldehydes, etc. Every year the need for propylene increases.

4.3.b. Addition of hydrogen halides (hydrohalogenation)
Another important reaction of electrophilic addition to alkenes is the long-known hydrohalogenation of alkenes.
The following are typical examples of the addition of HCl, HBr and HI to various alkenes.


The effect of alkyl substituents on the double bond on the addition rate is described by the following sequence:
R 2 C=CHR > RCH=CHR > RCH=CH 2
This is consistent with the mechanism in which the rate-determining step of the reaction is the formation of a carbocation, since the stability of alkyl cations decreases in the series tertiary > secondary > primary. Thus, the addition mechanism must involve the intermediate formation of either a free carbocation, which is rare, or an intermediate with a carbocationic character. The last case is the most typical.
If the addition occurred through a "free carbocation", then the reaction would be completely non-stereoselective, since alkyl cations have a planar structure. However, hydrohalogenation, as a rule, proceeds stereoselectively, and depending on the type of alkene, selective anti- connection, selective syn- or mixed syn-anti- accession.
For alkenes, in which the double bond is not conjugated with an aromatic ring, it is characteristic anti- addition of a hydrogen halide. Anti- addition of hydrogen chloride and hydrogen bromide, deuterium chloride and bromide is observed for cyclohexene, cyclopentene, 1,2-dimethylhexene, 1,2-dimethylpentene, cis- And trance-butene-2, hexene-3 and many other simple alkenes and cycloalkenes.

In the addition product, the same substituents (methyl groups) are located on opposite sides of the middle plane of the cyclohexane ring, therefore it belongs to trance-row. Anti-addition is hardly compatible with the mechanism in which the formation of a discrete carbocation is supposed. For a planar carbocation, the nucleophilic attack of the halide ion is equally probable on both sides of the plane, which should lead to the formation of a mixture of products syn- And anti- connections. The kinetics of alkene hydrohalogenation also points to a more complex addition mechanism. For non-conjugated alkenes, the reaction rate is described by a third order equation with a second order in terms of hydrogen halide, i.e., it corresponds to the Ad E 3 mechanism.
v = k [alkene] 2
The anti-addition and second order reaction with respect to the hydrogen halide is in good agreement with the Ad E 3 mechanism in which an alkene reacts with two hydrogen halide molecules, one of which acts as an electrophilic and the other as a nucleophilic agent.

Such a trimolecular mechanism suggests that a complex of an alkene and one hydrogen halide molecule is initially formed, followed by an attack by a second HX molecule on this complex with anti-sides without the formation of a discrete carbocation. It should be specially noted that any trimolecular mechanism must consist of two successive stages, since the simultaneous collision of three molecules is extremely unlikely.

Anti-addition indicates a preferential nucleophilic attack of the hydrogen halide from the side opposite to that from which the alkene is protonated. Instead of hydrogen halide, the function of the nucleophilic agent in the final stage can also be performed by the halide ion. Indeed, the reaction rate usually increases in direct proportion to the concentration of the halide ion introduced into the reaction mixture in the form of tetraalkylammonium halides NR 4+ X - or lithium LiX. In this case, stereospecific anti- accession.
For alkanes, in which the double bond is conjugated with an aromatic ring, it is characteristic syn- connection or mixed syn-anti-addition of a hydrogen halide, for example:

Syn-attachment is the dominant process for cis- And trance-isomers of 1-phenylpropene, 1-phenyl-4-alkylcyclohexenes, acenaphthylene, indene. The protonation of such alkenes results in the formation of benzyl-type carbocations, which are more stable than the pure alkyl cations resulting from the protonation of conventional alkenes and cycloalkenes. The reaction kinetics in this case is usually described by a simpler second-order equation v = k[alkene], i.e., it corresponds to the bimolecular Ad E 2 mechanism. The Ad E 2 mechanism involves the formation of an ion pair including a carbocation and a halide ion.

It cannot be expected that the addition mechanism involving ion pairs will be highly stereoselective. If the ion pair turns into the final product faster than the rotation around a simple carbon-carbon bond, the end result will be syn-addition, where a proton and a halide ion are attached on the same side of the double bond. Otherwise, the formation of products is observed as syn- so and anti- HX connections. Such a case is realized during hydrohalogenation pair-substituted styrenes Z-C 6 H 4 -CH=CH 2 . The pattern observed here is that syn-addition is typical only for those olefins that, when protonated, give a relatively stable carbocation, i.e., in the case of donor substituents Z.
Hydrohalogenation reactions proceeding according to the Ad E 2 mechanism are characterized by competition between the processes of conjugated addition and rearrangements, since a carbocation or an ion pair is formed as an intermediate.

As an example of rearrangements with 1,2-migration of an alkyl group and a hydride ion, we present the hydrohalogenation reactions of tert-butylethylene and isopropylethylene, respectively.

When carrying out the same reaction without a solvent in the cold (-78 0 C), a mixture of 33% normal and 67% anomalous (rearranged) addition products is formed.

4.3.c. Orientation. Markovnikov's rule
Unlike symmetrical electrophiles (Hal 2), hydrogen halides are unsymmetrical electrophilic reagents. The addition of any unsymmetrical electrophile (HBr, ICl, H 2 O, Hg (OAc) 2, etc.) to an unsymmetrical alkene could in principle give a mixture of two alternative products, but in practice only one of them is usually formed. As an example, consider the addition of hydrogen bromide to propylene.

Back in 1870, V.V. Markovnikov formulated an empirical rule according to which unsymmetrical alkenes add HX in such a way that a product is predominantly formed in which hydrogen is added to the least substituted, and X to the most substituted end of the double bond.
Usually, Markovnikov's rule is explained by the difference in stability of two alternative carbocations. For example, in the example above, the normal n-propyl cation is much less stable than isopropyl cation, and therefore the reaction proceeds along the second route.
Markovnikov's rule was originally used only for the addition of HX to hydrocarbon substrates, but in principle it can be extended to the reactions of other substituted alkenes. So, the addition of HCl to CF 3 CH=CH 2 gives " anti-Markovnikov "product CF 3 CH 2 CH 2 Cl. This was to be expected, since the cation CF 3 CH+ CH 3 is less stable than the cation CF 3 CH 2 CH 2+ due to the strong (-I)-effect of the CF 3 group. The cation CF 3 CH 2 CH 2+ is predominantly formed, but it is also, although to a lesser extent, destabilized by the inductive effect of CF 3 -group, as a result of which the addition of HCl to trifluoromethylethylene is much slower than the addition to unsubstituted ethylene.
For a similar reason, vinyltrialkylammonium cations add HBr also against the Markovnikov rule:
The addition of HX to alkenes with strong (-I) and (-M) substituents, for example, to acrylonitrile or nitroethylene, must also go against Markovnikov's rule. However, in this case the double bond is so strongly deactivated with respect to electrophilic reagents that these reactions proceed only under very severe conditions. Vinyl chloride CH 2 =CHCl always gives exclusively "Markovnikov adducts". For example, when it reacts with HCl, only 1,1-dichloroethane (geminal dichloride) CH 3 CHCl 2 is formed. Chlorine, similarly to the CF 3 group, has a strong (-I) effect, and at first glance, it seems that for this reason the addition should have an anti-Markovnikov orientation, since the + CH 2 CH 2 Cl cation should be more stable than the CH 3 CH + Cl cation. However, unlike the CF 3 group, chlorine, in addition to the (-I) effect, also has an (+ M) effect that counteracts it (because it has lone pairs). Experience shows that the magnitude of the mesomeric effect is quite sufficient to lower the energy of the 1-chloroethyl cation below the energy level of the 2-chloroethyl cation, in which the +M effect does not manifest itself.
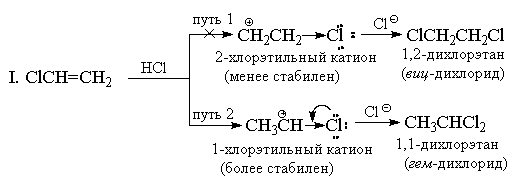
II. From the standpoint of resonance theory, the structure of the 1-chloroethyl cation can be represented as follows:
However, addition to vinyl chloride occurs more slowly than to ethylene under the same conditions, i.e., according to the total effect (-I> +M), chlorine remains an electron-withdrawing substituent compared to hydrogen, and the 1-chloroethyl cation is less stable than C 2 H 5 +. Other vinyl halides react similarly with HX.
Vinyl ethers CH 2 =CHOR add HX (X=Hal) according to Markovnikov's rule at a much higher rate than all the substituted alkenes listed above. This is due to the significant +M effect of the RO group. In contrast to the chlorine atom, the RO group in terms of the total electronic effect (+M > -I) is a strong electron-donating substituent, effectively stabilizing the neighboring carbocationic center. The structure of the carbocation in this case can also be represented as a set of two resonance structures
Attack of the oxonium cation with a halide anion leads to the formation of α-haloesters of the CH 3 CH(Hal)OR type.
4.3.d. Hydration of alkenes
Acid-catalyzed hydration of alkenes leads to the formation of alcohols. The direction of hydration of alkenes is determined by the Markovnikov rule, so it is assumed that a carbocation is formed as an intermediate particle in this reaction.
The tendency of secondary alkyl carbocations to rearrange themselves prevents the use of hydration of alkenes to obtain secondary alcohols.

This method in the laboratory has found a limited scope only for the preparation of tertiary alcohols. The hydration reaction in this case is largely reversible and tertiary alcohols are formed in low yields (40-45%).


Hydration of the simplest alkenes - ethylene and propylene - is an important industrial method for obtaining ethyl and isopropyl alcohols.

In laboratory practice, direct hydration of alkenes has not found wide application both because of harsh conditions and due to the formation of a significant amount of isomeric alcohols. At present, the related hydroxymercuration-demercuration reaction is commonly used for the regioselective production of alcohols from alkenes.
4.3.d. Hydroxymercuration-demercuration
Electrophilic attack on the double bond of an alkene can be carried out by metal ions, among which the mercury (II) cation occupies a special position. Mercury acetate under very mild conditions at 20 0 C adds to alkenes in aqueous tetrahydrofuran (THF) or in aqueous acetic acid to form organomercury compounds. The addition of mercury acetate to the double bond proceeds regiospecifically in strict accordance with Markovnikov's rule, i.e., the mercury cation attaches to the least substituted carbon atom.
The C-Hg bond in organomercury compounds can be easily cleaved by sodium borohydride NaBH 4 to form mercury and a new C-H bond. It is assumed that alkyl mercuric hydride is obtained as an unstable intermediate, which further decomposes with the release of metallic mercury by a radical mechanism.

In sum, this two-stage hydroxymercuration-demercuration process ultimately represents regiospecific alkene hydration according to Markovnikov's rule under extremely mild conditions, when the formation of by-products is reduced to the maximum possible minimum. This can be clearly illustrated by the following examples, in which the total yield of the reaction products is 90-98%. The given numerical data in this case do not indicate the yields of the compounds formed, but their ratio in the mixture.




As can be seen from the above examples, hydroxymercuration-demercuration of alkenes in most cases provides regiospecific hydration of alkenes with the formation of practically only one of the two isomeric alcohols. It should be noted that there is no need to isolate the organomercury compound, and both processes can be carried out directly one after the other.
Hydroxymercuration of unsymmetrical alkenes apparently begins with the attack of the AcOHg + cation and the formation of an unsymmetrical cyclic mercurinium cation (analogous to the unsymmetrical halonium ion) as an intermediate, which then opens as a result of a nucleophilic attack by water at the most substituted carbon atom bearing a larger positive charge.

The bridging mercury ion can be fixed in a non-nucleophilic strongly acidic medium even at 20 0 C by adding a stronger electrophilic agent - mercury trifluoroacetate in a mixture of fluorosulfonic acid and antimony pentafluoride.

The mercury cation can be split not only by the action of water, but also by other electron-donating solvents: alcohols, acetic acid, acetonitrile, etc. The final reaction product in this case will be ethers, acetates or N-substituted amides of acetic acid, respectively, for example:


When using branched secondary or tertiary alcohols in the alkoxymercuration-demercuration reaction, Hg(OCOCF 3) 2 trifluoroacetate or Hg(OSO 2 CF 3) 2 trifluoroacetate are more effective than mercury acetate.

Thus, hydroxy- and alkoxymercuration-demercuration is one of the best preparative methods for the synthesis of alcohols and ethers with branched alkyl radicals.
The addition of mercury salts to alkenes is the most striking example of a conjugated addition reaction to a double bond, where the role of the external nucleophilic agent is played by the solvent. The stereochemistry of the dual hydroxymercuration-demercuration process depends on the stereochemical outcome of each individual step. Hydroxymercuration is characterized by anti-addition, as for other reactions involving a cyclic cation. However, radical demercuration is not characterized by high stereoselectivity. Therefore, the whole process as a whole is also non-stereospecific.
4.3.f. Addition of alkenes (cationic dimerization and polymerization of alkenes)
The most interesting example of this type of reaction is the dimerization and polymerization of isobutylene in the presence of sulfuric acid.

The technical name for this mixture of alkenes is "diisobutylene". This reaction proceeds according to a cationic mechanism similar to the mechanism of addition of mineral acids to the double bond of alkenes. In the first step, a proton is attached to an isobutylene molecule to form a relatively stable tert-butylcation. Further formed tert-butylcation (Lewis acid) reacts with an isobutylene molecule (Lewis base) to form a new stable tertiary octyl cation.

Under these conditions, under the action of bases (H 2 O, HSO 4 - ions), the octyl carbocation quickly loses a proton and turns into a mixture of isomeric pentenes, since proton detachment occurs from two different positions:

The preferential formation of the thermodynamically less stable alkene - 2,4,4-trimethylpentene-1 (80% in the reaction mixture) is associated with a greater spatial accessibility for base attack of the hydrogen atoms of the methyl groups compared to the hydrogen atoms of the methylene group. In industry, "diisobutylene" is hydrogenated on Ni-Raney to produce "isooctane" (technical name for the hydrocarbon 2,2,4-trimethylpentane), used as a high-octane fuel for internal combustion engines.
At high concentrations of sulfuric acid (more than 80%), cationic polymerization of isobutylene occurs with the formation of a polymer called polyisobutylene (-CH 2 C(CH 3) 2 -) n . This rubber-like polymer is used to obtain anti-corrosion and waterproofing coatings, sealants, etc.
In addition to isobutylene, 3-methylbutene-1, vinyl ethers, and some styrene derivatives capable of forming relatively stable carbocations are polymerized according to the cationic mechanism. Hydrogen fluoride and Lewis acids are also used as catalysts for cationic polymerization: BF 3 , AlCl 3 , AlBr 3 and others in the presence of very small amounts of water.
4.3.g. Addition of alkanes (alkylation of alkenes)
Another industrial method for the synthesis of "isooctane" is based on the interaction of isobutylene with an excess of isobutane in the presence of concentrated sulfuric acid or in anhydrous hydrogen fluoride at 0 10 0 C.

This reaction also proceeds via the cationic mechanism and, what is of particular interest, is an example of a cationic chain process. First, isobutylene dimerizes under the reaction conditions to form a tertiary octyl cation (CH 3) 3 CCH 2 C + (CH 3) 2 . The mechanism of its formation is described in detail in the previous section. Next, there is a rapid transfer of hydrogen (in the form of a hydride ion) from isobutane to the octyl cation with the formation of an "isooctane" molecule and a new tert-butyl cation, which in turn quickly reacts with isobutylene to form a new octyl cation, etc.

In addition to the synthesis of "isooctane", this alkylation method is used in the petrochemical industry for the synthesis of high-boiling branched hydrocarbons from branched alkenes and low-boiling alkanes with a thermal cracking fraction.
ALKENES
Hydrocarbons, in the molecule of which, in addition to simple carbon-carbon and carbon-hydrogen σ-bonds, there are carbon-carbon π-bonds, are called unlimited. Since the formation of a π bond is formally equivalent to the loss of two hydrogen atoms by a molecule, unsaturated hydrocarbons contain 2p fewer hydrogen atoms than the limit, where P - number of π-bonds:
A series whose members differ from each other by (2H) n is called isological side. So, in the above scheme, the isologues are hexanes, hexenes, hexadienes, hexines, hexatrienes, etc.
Hydrocarbons containing one π-bond (i.e. double bond) are called alkenes (olefins) or, according to the first member of the series - ethylene, ethylene hydrocarbons. The general formula for their homologous series C p H 2n.
1. Nomenclature
In accordance with the rules of IUPAC, when constructing the names of alkenes, the longest carbon chain containing a double bond receives the name of the corresponding alkane, in which the ending -an changed to -en. This chain is numbered in such a way that the carbon atoms involved in the formation of a double bond receive the smallest number possible:

Radicals are named and numbered as in the case of alkanes.
For alkenes of relatively simple structure, simpler names are allowed. So, some of the most common alkenes are called by adding the suffix -en to the name of a hydrocarbon radical with the same carbon skeleton:
Hydrocarbon radicals formed from alkenes receive the suffix -enyl. The numbering in the radical starts from the carbon atom that has a free valency. However, for the simplest alkenyl radicals, instead of systematic names, it is allowed to use trivial ones:

Hydrogen atoms directly bonded to unsaturated carbon atoms forming a double bond are often referred to as vinyl hydrogen atoms,
2. Isomerism
In addition to the isomerism of the carbon skeleton, in the series of alkenes there is also the isomerism of the position of the double bond. In general, isomerism of this type - substituent position isomerism (functions)- is observed in all cases when there are any functional groups in the molecule. For alkane C 4 H 10, two structural isomers are possible:
For alkene C 4 H 8 (butene), three isomers are possible:

Butene-1 and butene-2 are isomers of the position of the function (in this case, its role is played by a double bond).
Spatial isomers differ in the spatial arrangement of substituents relative to each other and are called cis isomers, if the substituents are on the same side of the double bond, and trans isomers, if on opposite sides:

3. Double bond structure
The breaking energy of a molecule at the C=C double bond is 611 kJ/mol; since the energy of the σ-bond C-C is 339 kJ / mol, the energy of breaking the π bond is only 611-339 = 272 kJ / mol. π-electrons are much easier than σ-electrons to be influenced, for example, by polarizing solvents or by any attacking reagents. This is explained by the difference in the symmetry of the distribution of the electron cloud of σ- and π-electrons. The maximum overlap of p-orbitals and, consequently, the minimum free energy of the molecule are realized only with a planar structure of the vinyl fragment and with a shortened C-C distance equal to 0.134 nm, i.e. much smaller than the distance between carbon atoms connected by a single bond (0.154 nm). With the rotation of the "halves" of the molecule relative to each other along the axis of the double bond, the degree of overlapping of the orbitals decreases, which is associated with the expenditure of energy. The consequence of this is the absence of free rotation along the axis of the double bond and the existence of geometric isomers with the corresponding substitution at carbon atoms.
Alkenes- unsaturated hydrocarbons, which contain one double bond. Examples of alkenes:

Methods for obtaining alkenes.
1. Cracking of alkanes at 400-700°C. The reaction proceeds according to the free radical mechanism:

2. Dehydrogenation of alkanes:
3. Elimination reaction (cleavage): 2 atoms or 2 groups of atoms are cleaved off from neighboring carbon atoms, and a double bond is formed. These reactions include:
A) Dehydration of alcohols (heating above 150 ° C, with the participation of sulfuric acid as a water-removing reagent):
B) Cleavage of hydrogen halides when exposed to an alcoholic solution of alkali:

The hydrogen atom is split off mainly from the carbon atom that is associated with a smaller number of hydrogen atoms (the least hydrogenated atom) - Zaitsev's rule.
B) Dehalogenation:

Chemical properties of alkenes.
The properties of alkenes are determined by the presence of a multiple bond, therefore, alkenes enter into electrophilic addition reactions, which proceeds in several stages (H-X - reagent):
1st stage:

2nd stage:
 .
.
The hydrogen ion in this type of reaction belongs to the carbon atom that has a more negative charge. The density distribution is:

If there is a donor as a substituent, which manifests itself as an +I- effect, then the electron density shifts towards the most hydrogenated carbon atom, creating a partially negative charge on it. The reactions go along Markovnikov's rule: when attaching polar molecules of the type HX (HCl, HCN, HOH etc.) for unsymmetrical alkenes, hydrogen is added preferentially to the more hydrogenated carbon atom at the double bond.
A) Addition reactions:
1) Hydrohalogenation:
The reaction proceeds according to Markovnikov's rule. But if peroxide is present in the reaction, then the rule is not taken into account:
2) Hydration. The reaction proceeds according to Markovnikov's rule in the presence of phosphoric or sulfuric acid:

3) Halogenation. As a result, bromine water becomes decolorized - this is a qualitative reaction to a multiple bond:
4) Hydrogenation. The reaction proceeds in the presence of catalysts.



